* These authors contributed equally to this work.
1 Department of Oncology, Maoming People’s Hospital, 101 Weimin Road, Maoming, 525000, Guangdong, China.
✉ Correspondence: Jingyu Xie <hulinhui@live.cn>
Abstract
Objective
This study aimed to investigate the intriguing possibility of a causal relationship between the consumption of dried fruits and susceptibility to frailty using a thorough two-sample Mendelian randomization (MR) approach.
Methods
Utilizing data sourced from a comprehensive genome-wide association study (GWAS), encompassing 409,125 individuals with recorded dried fruit consumption and 175,226 cases exhibiting frailty, we meticulously selected 41 genetic loci as instrumental variables closely linked to dried fruit consumption. Employing MR, we conducted analyses via random effects inverse variance weighting (IVW), the weighted median method, and Mr-Egger methods. Additionally, sensitivity analyses were conducted using the Mr-Egger test to detect potential pleiotropy and leave-one-out analysis.
Results
In the IVW analysis, we observed a significant negative correlation between dried fruit consumption and frailty (β = -0.030, SE = 0.115, p = 0.001). The weighted median method yielded consistent results (β = -0.446, SE = 0.110, p = 0.000). While the Mr-Egger analysis showed a wider confidence interval (β = -0.430, SE = 0.515, p = 0.409), no evidence of pleiotropy was detected. Heterogeneity was evident, as indicated by Cochran’s Q statistic, which detected significant variability in SNP effects (all p < 0.001). However, the leave-one-out analysis and funnel plot demonstrated the robustness of our findings without any single SNP driving these associations.
Conclusion
This comprehensive Mendelian randomization study provides compelling evidence supporting a potentially negative causal relationship between dried fruit consumption and the incidence of frailty, indicating its relevance as a critical area for further investigation.
Keywords
frailty; dried fruit consumption; Mendelian randomization
INTRODUCTION
Frailty constitutes a multifaceted physiological and clinical syndrome, frequently characterized by a gradual deterioration across various domains of bodily function, encompassing the immune system and cardiovascular health, among others. This constellation of factors renders individuals more susceptible to illnesses and external stressors (Hoogendijk et al., 2019). The ramifications of frailty extend beyond mere health concerns, profoundly impacting the quality of life for older adults and heightening the risk of adverse consequences such as falls, fractures, hospitalizations, and mortality. Furthermore, it imposes a substantial burden on healthcare systems and the socioeconomic landscape (Veronese et al., 2021).
Hence, delving into the intricate mechanisms of frailty and identifying potential interventions to ameliorate or delay its onset holds paramount importance for public health and clinical practice. Dietary patterns have a direct nexus with frailty. Diet represents a pivotal determinant in maintaining overall health and functionality, and dried fruits emerge as a salubrious dietary choice, brimming with vitamins, minerals, fiber, and antioxidants, believed to bolster normal bodily functions (Ceglia et al., 2023; Wang et al., 2023). Moreover, dried fruits may exert a favorable influence on cardiovascular health and the inflammatory response, both inextricably linked with frailty development (Sullivan et al., 2020).Evidently, studies have ascertained that individuals adhering to diets enriched with dried fruits tend to exhibit superior cardiovascular health, lower blood pressure levels, and diminished inflammatory markers (Wang et al., 2021; Zeng et al., 2023). These attributes closely intertwine with frailty development, given that cardiovascular health, immune system functionality, and inflammatory states all wield influence over frailty progression (Rosado-Pérez et al., 2023).Nonetheless, the causal relationship between fruit consumption and frailty remains a subject of contention. Numerous observational investigations have intimated a correlation between dried fruit consumption and reduced frailty risk, but these studies are frequently impeded by methodological constraints and potential confounding variables (Gavia-García et al., 2023). Furthermore, substantiating the causality of this association necessitates the availability of comprehensive, long-term clinical trial data.
Enter the Mendelian randomization (MR) method, a potent tool in epidemiological research. Its core premise revolves around leveraging genetic variability as a means to elucidate causal connections between risk factors and specific maladies (Titova et al., 2020; Ahmed et al., 2021). In the realm of epidemiological studies, confounding factors cast long shadows over causal inferences. In contrast, Mendelian randomization studies harness genetic variation, mirroring the randomized allocation of alleles to offspring akin to randomized controlled trials (Cho S et al., 2021). This approach adeptly sidesteps confounding variables, mitigates the perils of reverse causality seen in observational studies, and bestows the advantages of representativeness and feasibility akin to randomized controlled trials (Birney et al., 2022).
Regrettably, Mendelian randomization studies probing the association between fruit consumption and frailty remain conspicuously absent. Consequently, the primary objective of this study resides in exploring the causal nexus between dried fruit consumption and frailty via the robust tool of Mendelian randomization (MR), which has the potential to furnish more compelling causal evidence. Our underlying hypothesis posits that genetic variation can be employed as an instrumental variable for fruit consumption to gauge its impact on frailty risk. By embracing the MR approach, we aspire to deliver a more precise assessment of the causal interplay between dried fruit consumption and frailty. This, in turn, could pave the way for more targeted recommendations in the domains of public health policies and healthcare guidelines, ultimately fostering an enhanced quality of life for older adults while mitigating frailty-associated adverse outcomes.
METHODS
Study design
In this investigation, we harnessed the consolidated dataset from a genome-wide association study (GWAS) to execute a two-sample Mendelian randomization (MR) analysis, aimed at scrutinizing the potential causal linkage between dried fruit consumption and the incidence of frailty. To fortify the robustness of our findings, we subjected them to a rigorous sensitivity analysis.It is imperative to underscore that Mendelian randomization inquiries necessitate the meticulous fulfillment of three fundamental assumptions: association, independence, and exclusivity. Specifically, these assumptions dictate that: (1) the instrumental variables must exhibit a robust correlation with the exposure factors under consideration; (2) these instrumental variables should remain devoid of any correlations with confounding elements that might be intertwined with the “exposure-outcome” relationship; (3) the instrumental variables exert their influence on the outcome variables exclusively through the mediation of the exposure factors. Please refer to Figure 1 for a graphical representation of this conceptual framework.

Data source
The primary data source for our investigation was predominantly derived from the UK Biobank, accessible via the Mr-Base platform. This encompassed a comprehensive dataset comprising 409,125 instances of dried fruit consumption (Pirastu et al., 2022). Moreover, pertinent data pertaining to frailty cases were also extracted from the UK Biobank, encapsulating a substantial cohort of 175,226 cases (Atkins et al., 2021). Detailed specifics are available in Table 1. It is noteworthy that all data were procured from publicly accessible repositories or previously conducted studies. Importantly, no supplementary informed consent procedures were necessitated.
| variables | ID | Sample size | Number of SNPs | Population | Sex | Year |
|---|---|---|---|---|---|---|
| Dried fruit consumption | ebi-a-GCST90096909 | 409125 | / | European | Men and women | 2022 |
| Frailty | ebi-a-GCST90020053 | 175226 | 7589717 | European | Men and women | 2021 |
Instrumental variables
Initially, in accordance with the primary Mendelian randomization (MR) hypothesis necessitating a robust linkage between single nucleotide polymorphisms (SNPs) and dried fruit consumption, we meticulously identified SNPs exhibiting a substantial genome-wide association with dried fruit consumption (P < 5×10-8, R2 < 0.001, genetic distance = 10,000 kilobases).Subsequently, to fulfill the secondary MR hypothesis asserting that genetic variance should remain unrelated to potential confounding variables, an inquiry was conducted within the Phenoscanner database. This inquiry ascertained that the selected SNPs were devoid of associations with recognized confounders. Ultimately, instrumental variables were derived from SNPs that exhibited significant correlations with dried fruit consumption, employing a rigorous heterogeneity test to exclude markedly heterogeneous SNPs.The veracity of the association hypothesis was further assessed by scrutinizing the presence or absence of weak instrumental variable bias concerning the chosen instrumental variable. This evaluation relied on the computation of the F statistic, with an F value exceeding 10 signifying an absence of weak instrumental variable bias. The formula for F calculation is as follows: F=[(N-K-1)/K]×[R2/(1-R2)], where N represents the sample size of the exposure factor, K signifies the count of instrumental variables, and R2 corresponds to the proportion of variation in the exposure factor explicable by the instrumental variable.
Mendelian randomization
Within this investigation, we employed a comprehensive array of analytical approaches to scrutinize the causal relationship between dried fruit consumption and the incidence of frailty through two-sample Mendelian randomization. The principal methodologies encompassed random effects inverse-variance weighted (IVW), weighted median, and Mr-Egger methods.Notably, we recognized that conventional inverse variance weighted analysis might be susceptible to distortions arising from invalid instrumental biases or pleiotropic effects. Consequently, we judiciously incorporated sensitivity analyses to meticulously evaluate the validity and robustness of our IVW-derived results.Furthermore, to mitigate the potential influence of horizontal pleiotropy, which can confound outcomes via causal pathways unrelated to the exposure, we extended our investigation by employing the weighted median and Mr-Egger regression techniques to assess the association between dried fruit consumption and frailty.In our pursuit of unwavering rigor, results that achieved statistical significance were subjected to additional assessments of heterogeneity. This multifaceted evaluation involved the Mr-Egger intercept test, sensitivity analyses, and modified Cochran Q statistics.It’s imperative to emphasize that all statistical tests adhered to a two-tailed framework, ensuring the comprehensiveness of our analyses.
Results
Determination of instrumental variables and judgment of bias of weak instrumental variables
Following the exclusion of instrumental variables exhibiting continuous imbalance, a judicious curation process yielded a selection of 41 Single Nucleotide Polymorphisms (SNPs) that exhibited significant associations with dried fruit consumption as ascertained through the GWAS study (R2<0.001, P<5×10-8). It is noteworthy that all the SNPs included in this investigation boasted F statistics exceeding the threshold of 10. This compelling observation indicates the absence of weak instrumental variable bias within our study, thereby reinforcing the robustness and reliability of our findings.To delineate the impact of these SNPs on frailty, we conducted Mendelian randomization analysis involving two independent samples. A detailed illustration of these results is thoughtfully presented in Figure 2 for comprehensive elucidation.

The effect of dried fruit consumption on the risk of frailty
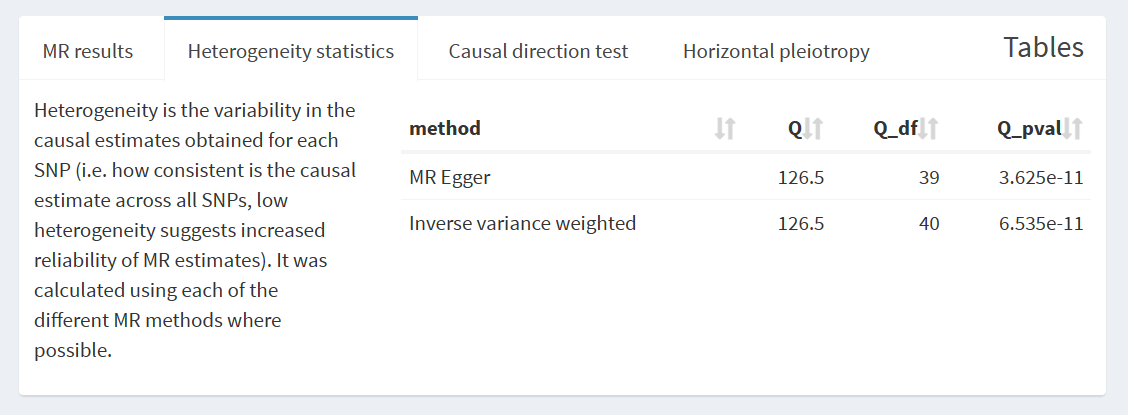
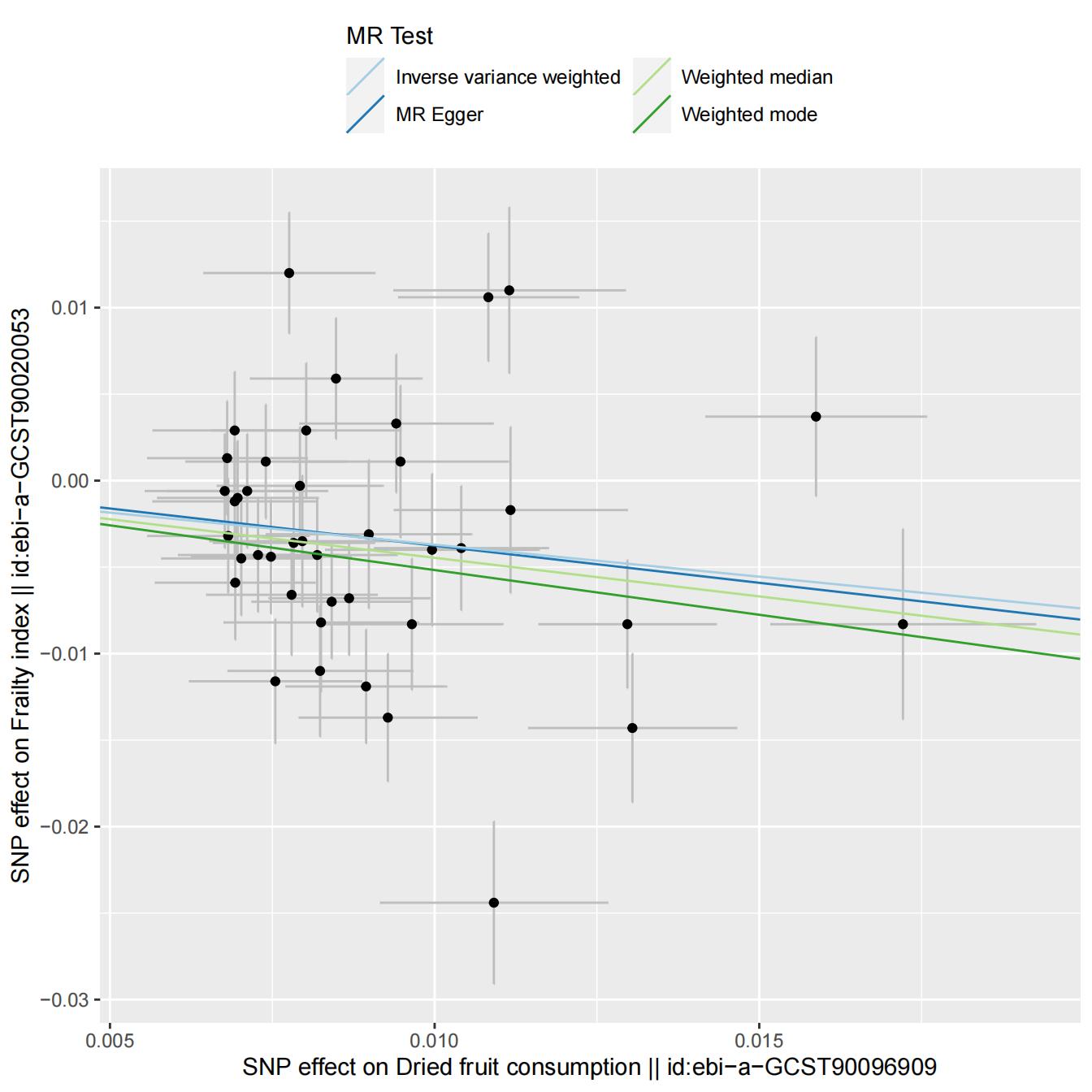
As meticulously depicted in Table 2 and Figure 3, our genetic prediction outcomes substantiate a close and noteworthy correlation between dried fruit consumption and the manifestation of frailty. Employing the Inverse-Variance Weighting (IVW) approach, we observed a statistically significant negative association between fruit consumption and frailty (β=-0.030, SE = 0.115, p =0.001). Encouragingly, the Weighted Median method produced strikingly akin results (β=-0.446, SE = 0.110, p =0.000). Notably, the MR Egger method, while exhibiting a broader range of results (β=-0.430, SE = 0.515, p =0.409), did not detract from the overall coherence of our findings.Our analytical journey unveiled evidence of heterogeneity, yet conspicuously absent was any indication of pleiotropy. Remarkably, Cochran’s Q statistic sounded the clarion, detecting significant heterogeneity amongst SNP effects (all p <0.001). In our quest for comprehensiveness, a leave-one analysis was conducted, affirming the absence of any singular SNP wielding disproportionate influence in driving these associations, as elucidated in Figure 4. Furthermore, an assuring exploration of the funnel plot in Figure 5 revealed an absence of any discernible drift within our study.
| MR method | Number of SNPs | Beta | SE | ssociation p value | Cochran Q statistic | Heterogeneity p value |
|---|---|---|---|---|---|---|
| Inverse variance weighted | 41 | -0.370 | 0.115 | 0.001 | 126.5 | 6.535e-11 |
| MR Egger | 41 | -0.430 | 0.515 | 0.409 | 126.5 | 3.625e-11 |
| Weighted median | 41 | -0.446 | 0.110 | 0.000 | / | / |


Discussion
This investigation employed Mendelian randomization as a systematic framework to scrutinize the causal nexus between genetic predisposition to dried fruit consumption and the jeopardy of frailty. The genetic prediction outcomes elegantly unveil a substantial association, showcasing that increased dried fruit consumption aligns with a diminished frailty risk. This association was further buttressed by sensitivity analyses, firmly affirming the inverse relationship between these two variables. Remarkably, our findings harmonize with antecedent observational studies (Wang et al., 2023).Of paramount importance, it warrants emphasis that genetic variation remains unwavering throughout an individual’s lifespan, and alleles are randomly allocated and stabilized. Thus, our study outcomes resonate, imparting credence to the concept that fruit consumption acts as a deterrent against frailty risk while adeptly sidestepping the quagmire of confounding factors and reverse causality. Naturally, it is prudent to acknowledge the presence of certain limitations in our study. Foremost amongst these challenges is the specter of horizontal pleiotropy, wherein genetic variations wield influence over outcomes via pathways distinct from the primary exposure of interest. Although we harnessed an extensive array of genetic variants linked to both dried fruit consumption and frailty as potent instruments, the intricate characterization of these variants remained elusive. Nevertheless, our tenacity to address these potential concerns is exemplified by the meticulous inclusion of a multifaceted sensitivity analysis, which harnessed a battery of pleiotropic-robust MR techniques, yielding largely concordant outcomes. Furthermore, it merits notation that our study cohort exclusively comprised populations of European ancestry, hence, constraining our ability to expound potential genetic distinctions across diverse ethnicities, nations, and regions. Lastly, owing to the scarcity of granular clinical data, our study refrained from executing subgroup analyses aimed at elucidating specific causal associations.
In summation, this inquiry augments our understanding of the intricate interplay between fruit consumption and frailty risk, underscoring the pivotal role of dietary proclivities in the realm of health. These findings proffer valuable insights that could effectively inform public health policies and tailor individualized health management recommendations, ultimately curbing the perils of frailty and its attendant health adversities.
References
Hoogendijk, E. O., Afilalo, J., Ensrud, K. E., Kowal, P., Onder, G., & Fried, L. P. (2019). Frailty: implications for clinical practice and public health. Lancet (London, England), 394(10206), 1365–1375. https://doi.org/10.1016/S0140-6736(19)31786-6
Veronese, N., Custodero, C., Cella, A., Demurtas, J., Zora, S., Maggi, S., Barbagallo, M., Sabbà, C., Ferrucci, L., & Pilotto, A. (2021). Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing research reviews, 72, 101498. https://doi.org/10.1016/j.arr.2021.101498
Ceglia, L., Shea, K., Rasmussen, H., Gilhooly, C. H., & Dawson-Hughes, B. (2023). A Randomized Study on the Effect of Dried Fruit on Acid-Base Balance, Diet Quality, and Markers of Musculoskeletal Health in Community Dwelling Adults. Journal of the American Nutrition Association, 42(5), 476–483. https://doi.org/10.1080/27697061.2022.2082599
Wang, Y., Haskell-Ramsay, C., Gallegos, J. L., & Lodge, J. K. (2023). Effects of chronic consumption of specific fruit (berries, cherries and citrus) on cognitive health: a systematic review and meta-analysis of randomised controlled trials. European journal of clinical nutrition, 77(1), 7–22. https://doi.org/10.1038/s41430-022-01138-x
Sullivan VK, Petersen KS, Kris-Etherton PM. Dried fruit consumption and cardiometabolic health: a randomised crossover trial. Br J Nutr. 2020;124(9):912-921. doi:10.1017/S0007114520002007
Wang, Y., Gallegos, J. L., Haskell-Ramsay, C., & Lodge, J. K. (2021). Effects of chronic consumption of specific fruit (berries, citrus and cherries) on CVD risk factors: a systematic review and meta-analysis of randomised controlled trials. European journal of nutrition, 60(2), 615–639. https://doi.org/10.1007/s00394-020-02299-w
Zeng, Y., Cao, S., & Yang, H. (2023). Causal associations between dried fruit intake and cardiovascular disease: A Mendelian randomization study. Frontiers in cardiovascular medicine, 10, 1080252. https://doi.org/10.3389/fcvm.2023.1080252Zeng,
Y., Cao, S., & Yang, H. (2023). Causal associations between dried fruit intake and cardiovascular disease: A Mendelian randomization study. Frontiers in cardiovascular medicine, 10, 1080252. https://doi.org/10.3389/fcvm.2023.1080252
Rosado-Pérez, J., Aguiñiga-Sánchez, I., Santiago-Osorio, E., & Mendoza-Núñez, V. M. (2019). Effect of Sechium edule var. nigrum spinosum (Chayote) on Oxidative Stress and Pro-Inflammatory Markers in Older Adults with Metabolic Syndrome: An Exploratory Study. Antioxidants (Basel, Switzerland), 8(5), 146. https://doi.org/10.3390/antiox8050146
Gavia-García, G., Rosado-Pérez, J., Aguiñiga-Sánchez, I., Santiago-Osorio, E., & Mendoza-Núñez, V. M. (2020). Effect of Sechium edule var. nigrum spinosum (Chayote) on Telomerase Levels and Antioxidant Capacity in Older Adults with Metabolic Syndrome. Antioxidants (Basel, Switzerland), 9(7), 634. https://doi.org/10.3390/antiox9070634
Titova, O. E., Michaëlsson, K., & Larsson, S. C. (2020). Sleep Duration and Stroke: Prospective Cohort Study and Mendelian Randomization Analysis. Stroke, 51(11), 3279–3285. https://doi.org/10.1161/STROKEAHA.120.029902
Ahmed, M., Mulugeta, A., Lee, S. H., Mäkinen, V. P., Boyle, T., & Hyppönen, E. (2021). Adiposity and cancer: a Mendelian randomization analysis in the UK biobank. International journal of obesity (2005), 45(12), 2657–2665. https://doi.org/10.1038/s41366-021-00942-y
Cho S. Long-Term Relationships Between Negative Life Events and Suicidal Ideation Specifying Agnew’s General Theory of Crime and Delinquency: A 7-Year Longitudinal Mediation Analysis. Violence Vict. 2023;38(4):459-484. doi:10.1891/VV-2021-0130
Birney E. (2022). Mendelian Randomization. Cold Spring Harbor perspectives in medicine, 12(4), a041302. https://doi.org/10.1101/cshperspect.a041302
Pirastu N, McDonnell C, Grzeszkowiak EJ,Mounier N, Imamura F, Merino J, et al. (2022)Using genetic variation to disentangle the complexrelationship between food intake and healthoutcomes. PLoS Genet 18(6): e1010162. https://doi.org/10.1371/journal.pgen.1010162
Atkins, J. L., Jylhävä, J., Pedersen, N. L.,Magnusson, P. K., Lu, Y., Wang, Y., Hägg, S., Melzer, D.,Williams, D. M., & Pilling, L. C. (2021). A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell, 20, e13459. https://doi.org/10.1111/acel.1345914749726
辅助材料
在线文档
中文手稿:【金山文档】 两样本孟德尔随机化研究干果消费与虚弱 发生风险的因果关系 https://kdocs.cn/l/cngAr5dAuzLb
原始手稿:【金山文档】 Causal association between Dried fruit consumption and the Risk of frailty two sample Mendelian randomization study https://kdocs.cn/l/cuYW8OZ7BvI8
Figure 1: 【金山文档】 孟德尔关系图 https://kdocs.cn/l/ctk0S1IfDC0f
Figure 2: 核苷酸分析.pdf
Figure 3: 多效分析图.pdf
Figure 4: 留一法图.pdf
Figure 5: 漏斗图.pdf
备用
辅助图片
网站来源:http://app.mrbase.org/